Diploma in Clinical research (CR) course
Clinical Research
Diploma in Clinical Research Course is an applied research discipline and involves Clinical Trials. CR deals with the safety and effectiveness (efficacy) of medications, devices, diagnostic products and treatment regimens for prevention, treatment, diagnosis or for relieving symptoms of a certain medical condition or disease under question involving volunteers or human beings used as subjects. Clinical trials are the last part of the clinical research process which is conducted in various phases. They are the predominant way that scientists find out if a new treatment or drug or device is safe and effective in people. A clinical trial is used often to discover and learn if a new treatment is more effective or not and has side effects than the standard treatment
Clinical Trial in the Drug Developement Process

Clinical Research Industry

India has become one of the foremost global destinations for clinical trials recently. CR is an attractive and lucrative industry for all medical and health care professions Globally and India specially because it foresees the tremendous growth and job opportunities because of its growing demand in the drug discovery and development process The Indian Clinical Research Industry is a very essential part of the pharmaceutical sector at an International Platform to provide a great economic support to the nation. According to the World Health Organization’s (WHO) report on pharmaceutical consumption, medicines to treat chronic diseases accounted for a larger volume of drug consumption in hospitals and non-hospitals set ups. Henceforth large volume of medicines is made available to healthcare consumers. Growing demand of drugs have made this Clinical trials prospects reach a higher and greater scale thereby serving this market with lucrative opportunities.
Clinical trials and other related services are outsourced now by all big pharma companies to reduce cost and to minimize operational expenses. This serves as an opportunity in developing regions like India to gain more revenue share. Manufacturers are now focusing on remodeling Product development processes are remodeled by manufacturers for the well-being of the consumer’s needs across the globe.
The six month Diploma programme or curriculum emphasizes precised and skilled learning. The blended –learning approach includes face to face lectures, interactive sessions, and webinars including intensive in person workshop. The curriculum is designed and developed by Drug information association (DIA). DIA being our content developer offers a strong linkage program, to making industry ready manpower. Founded in 1964, DIA is an independent, non-profit organization headquartered in Washington, D.C., USA with regional offices covering North and South America (Horsham, Pennsylvania, USA); Europe, North Africa and the Middle East (Basel, Switzerland); Japan (Tokyo); India (Mumbai); and China (Beijing).DIA along with DCS develops and implements educational programmes in professional trainings for domain expertise in the Clinical Research industry maintaining the highest standards for credibility and integrity.
This 6 month course provides industry exposure apart from the benefits listed below:
- Better industrial training and skills with detailed knowledge of the standards of their work.
- Both practical and professional training on the regulations and work processes of the Industry
- Good hold on the framework of Clinical Research industry leading to availability of best employment options.
- Accreditation with International Certification to be valued across the globe.
- Domain expertise and getting hands on practical exposure on various tools and software’s.
- Internship & placement to all successful students
Based upon ICH guidelines and Good pharmacovigilance practices (GVP) this course provides a complete training solution to acquire and expertise pharmacovigilance knowledge.
Be acquaint with Global Pharmacovigilance regulations in various countries including ICH regulations for drug safety and pharmacovigilance
Proficiency in handling Oracle Clinical/Rave/MedDRA
Skilled and proficient in presentation, writing, communication, Interview ,analytical and other soft skill aspects
Become industry ready professionals with advanced knowledge of the roles and responsibilities of various functionaries in Clinical Research industry like Clinical data management Specialist, Lab Programmers, Study validation expert/Study builders as per industry standards.
Become well aligned with industrial requirements as well as regulatory requirements to help and protect human kind.
- Doctors (MBBS/ MD/ BDS/ BAMS/ BHMS)
Pharma Candidates (B. Pharm / M. Pharm)
Life Sciences (M.Sc. / B.Sc)
- Credits/sessions: 48
- hours per session/credit: 3/5 in weekdays or weekends respectively
After 3-4 months of the course as per students’ performance and scorecard.
In Placement Preparation Program, DCS trains all students for facing interview and selection process or procedures of the corporate world like Writing Skills Communication Skills Interview Skills and other aspects.100% placement assured for all successful candidates per students’ performance and scorecard
Career prospects upon completion and certification of the course
Enormous job opportunities are booming in the clinical research field today and for the future ahead .The demand is growing continuously along with the requirement of drug development and disease management. Clinical trials and Clinical research deals with medical knowledge related to the treatment, diagnosis and prevention of the diseases or conditions being studied with clinically meaningful data for disease management and also to evaluate the safety and efficacy of the drug which will be or being used for a particular disease thereby ensuring the well-being of the public health.
Clinical trials require clinical research coordinators (CRCs) and clinical research associates (CRAs) to ensure everything is accordance with ICH guidelines and Good Clinical practices apart from investigators/doctors/physicians who investigate the effects of drug therapies on patient populations.
A Clinical Research professional starts with approx 3 Lakhs salary package per anum and eventually the monetary part reaches high with knowledge ,expertise, position, designation as much as 10-15 Lakhs per anum or more than that :
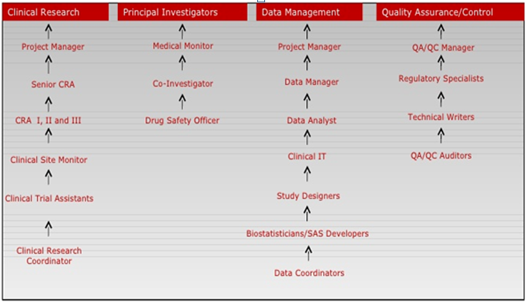
Top players in the Clinical research Industry are
Contract Research Organizations(CROs),Clinical Laboratories, Government Sectors, Biotechnology labs, Pharmaceuticals Companies, Pharmacovigilance industry, IT companies in aligned with Healthcare Industries, Hospitals, Regulatory Affairs Trial /Site management Organizations(TMO/SMO),Research and development Industries
















































