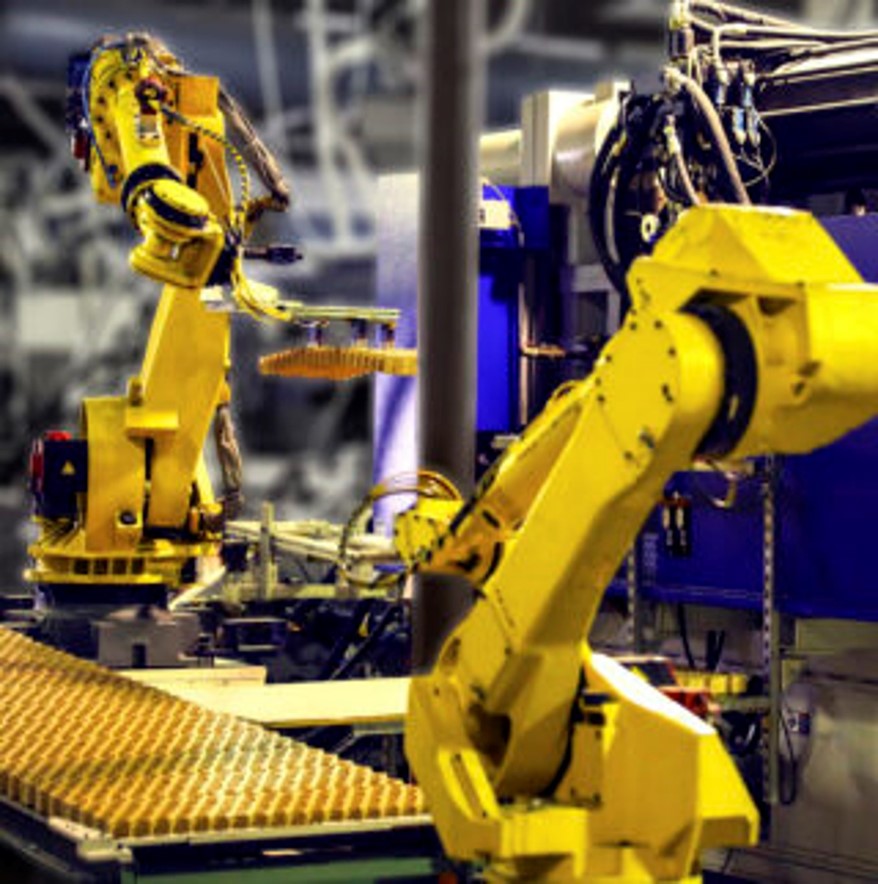
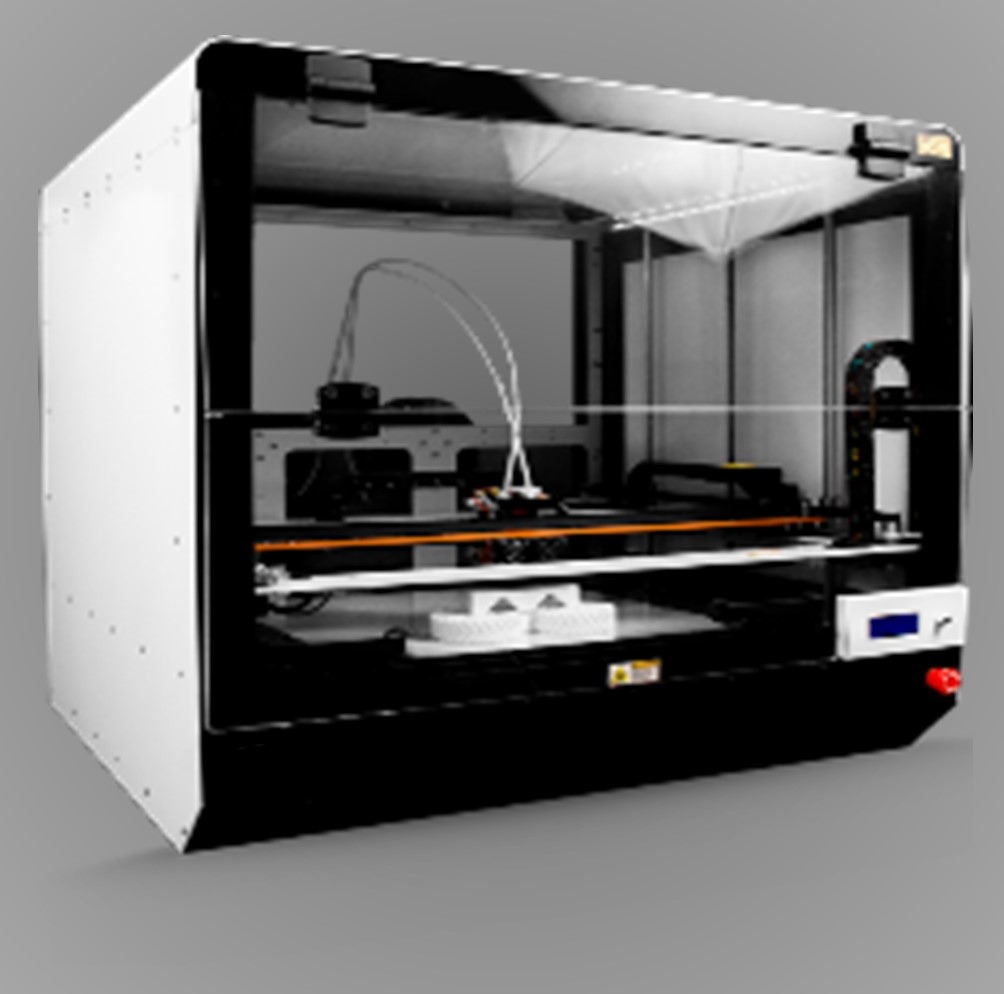
DCS offers a comprehensive suite of services in Smart Manufacturing designed to elevate operational efficiency and innovation. Their solutions integrate advanced automation technologies, real-time data analytics, and industrial IoT applications. Key services include Computer-Aided Design (CAD) for precise and efficient product development, and Computer Numerical Control (CNC) for accurate and automated machining processes. DCS also specializes in adaptive manufacturing techniques that allow for flexible and responsive production systems, as well as reverse engineering to analyze and improve existing components. Additionally, their expertise in robotics facilitates advanced automation and enhances productivity on the shop floor. By leveraging these technologies, DCS helps manufacturers optimize production processes, reduce downtime, and improve product quality in a rapidly evolving industry landscape.
Ministry of Health, Labour and Welfare
The Ministry of Health, Labour and Welfare is a cabinet-level agency in Japan.
Read More
AI and Machine Learning
Predictive Manufacturing uses data analytics to forecast and optimize production processes.
Read More
IIoT and Connectivity
IIoT enables real-time connectivity, enhancing smart manufacturing and operational efficiency.
Read More